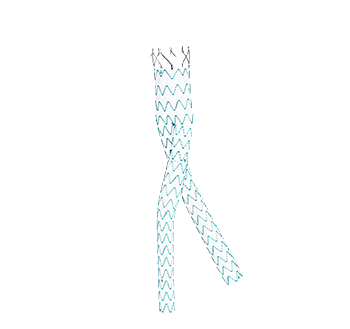
Percutek AAA Stent Graft

- Optimal M-shaped proximal stents allow durable seal and excellent circumferential conformability
- Single-suturing design offers great flexibility
- Nitinol alloy helps balance between strong radial force with excellent conformability
- Anchoring pins on the suprarenal stent provide active fixation without device migration
- Kink-resistant and low-profile delivery system (16Fr for 22mm and below stent grafts) with coverage hydrophilic coating offer smooth delivery
- Equal-sign shaped markers provide precise positioning
- Post-release mechanism assures well controlled deployment
- Adjustable re-positioning even after up to 3 stents deployment
- Three-piece design allows flexible and adjustable overlapping options
- Optimal M-shaped proximal stents and single-suturing design provide great flexibility and excellent conformability, allowing shortest proximal landing zone (10mm) and largest infrarenal angulation (75º) requirements
Configuration- Main body
|
Proximal graft diameter |
Distal graft diameter |
Total covered length |
OD of delivery system |
|
20mm/22mm |
12mm |
110mm |
16Fr |
|
24mm |
12mm |
110mm |
18Fr |
|
26mm/28mm |
14mm |
110mm |
18Fr |
|
30mm/32mm/34mm/36mm |
14mm |
110mm |
20Fr |
Configuration- Limb
|
Proximal graft diameter |
Distal graft diameter |
Total covered length |
OD of delivery system |
|
16mm |
10mm/13mm/16mm |
60mm/80mm/95mm/110mm/125mm/140mm/155mm |
14Fr |
|
16mm |
16mm |
155mm |
16Fr |
|
16mm |
20mm |
60mm/80mm/95mm/110mm/125mm/140mm/155mm |
16Fr |
|
16mm |
24mm |
60mm/80mm/95mm/110mm/125mm/140mm/155mm |
18Fr |
* exception- 16Fr, instead of 14Fr, applies to PAIL1616155
Configuration- Iliac extension
|
Proximal graft diameter |
Distal graft diameter |
Total covered length |
OD of delivery system |
|
10mm |
10mm |
80mm |
14Fr |
|
13mm |
13mm |
80mm |
14Fr |
|
20mm |
20mm |
80mm |
16Fr |
|
24mm |
24mm |
80mm |
18Fr |
Configuration- PAUI
|
Proximal graft diameter |
Distal graft diameter |
Total covered length |
OD of delivery system |
|
18mm/20mm/22mm |
14mm |
110mm |
16Fr |
|
24mm/26mm/28mm |
14mm |
110mm |
18Fr |
|
30mm/32mm/34mm/36mm |
14mm |
110mm |
20Fr |
Configuration- Cuff
|
Proximal graft diameter |
Distal graft diameter |
Total covered length |
OD of delivery system |
|
20mm/22mm |
20mm/22mm |
45mm/70mm |
16Fr |
|
24mm/26mm/28mm |
24mm/26mm/28mm |
45mm/70mm |
18Fr |
|
30mm/32mm/34mm/36mm |
30mm/32mm/34mm/36mm |
45mm/70mm |
20Fr |
The clinical trial of Percutek's Abdominal Aortic Stent Graft System was an open-label, non-randomized, concurrent controlled clinical trial.
A total of 153 patients were enrolled in 11 sites, including 82 patients in the experimental group and 71 patients in the control group.
The primary effectiveness endpoint was the percentage of patients who were successfully treated for abdominal aneurysms. The success rate of was 95.8% in the experimental group and 90.6% in the control group.
Table 1 Success rate of abdominal aneurysm treatment (adjusted PPS set):
|
Experimental group (%, m/n) |
Control group (%, m/n) |
|
|
Success rate |
96.0% (72/75) |
91.0% (61/67) |
The primary safety endpoint was the percentage of patients who had no major adverse events during the perioperative period. 97.5% of patients in the experimental group had no major clinical adverse events within 30 days (perioperative period), compared with 97.0% of patients in the control group.
Table 2 Safety evaluation:
|
Experimental group (%, m/n) |
Control group (%, m/n) |
|
|
Rate of freedom from major adverse events within 30 days |
97.5% (79/81) |
97.0% (65/67) |